The Application of Warning Labels in Medical Products?

In medical product design, warning labels are not only a rigid requirement for regulatory compliance but also serve as the "last line of defense" for user safety. As an industrial design team deeply rooted in the medical field, we understand that excellent warning design requires a balance between functional warnings, cognitive efficiency, and emotional experience. This article will begin with design practices to analyze the application logic and innovative directions of medical warning labels.
Outline of this issue:
· Definition of warning labels for medical products
· The value of warning labels in medical scenarios
· Industrial design principles for medical product warning labels
(Definition of Warning Labels for Medical Products)
Warning labels in medical products refer to mandatory informational markers that use visual or tactile forms such as text, symbols, graphics, and colors to clearly indicate potential risks, usage restrictions, operating specifications, or safety reminders of the product. Their core purpose is to mitigate usage risks, ensure the safety of patients and operators, and meet regulatory compliance requirements.

(Value of Warning Labels in Medical Scenarios)
· Risk Classification and Immediate Intervention
Risk Classification Framework: Based on Severity of Harm and Probability of Occurrence
The following references ISO 14971 (Medical Device Risk Management) and AAMI HE75 (Human Factors Engineering Design Guidelines), aligning differentiated label designs with intervention mechanisms.
| Risk Level | Definition | Design Response |
|---|---|---|
| Level I (Emergency) | May result in death or permanent injury | Red + Flashing Symbol + Continuous Beep |
| Level II (Severe) | May result in functional loss or severe injury | Yellow + Static Warning Symbol + Intermittent Alarm |
| Level III (Warning) | Reversible injury or operational delay | Orange/Blue + Text Prompt + Single Alert Tone |
| Level IV (Prompt) | Operational assistance information or status prompt | Green/Gray + Text Only/Icon |
· Tangible Expression of Human Factors Engineering
The tangible expression of human factors engineering in medical device warning labels essentially involves "translating" risks into users' instinctive responses. Based on the physiological, psychological, cognitive habits, and operational scenarios of users (healthcare professionals, patients, and their families), abstract safety risks are transformed into intuitive and perceptible warning signals through multidimensional design in visual, tactile, auditory, and interactive aspects.
WYYL Design's Cognitive Stratification of Users:
| User Type | Cognitive Characteristics | Design Strategy |
|---|---|---|
| Professional Healthcare Workers | High-pressure environment / Rapid decision-making | High-contrast symbols + Spatial memory reinforcement |
| Elderly Patients | Declining visual acuity / Slower reaction time | Tactile feedback + Gradient auditory cues |
| Non-Professional Users | Limited medical knowledge | Simplified language + Intuitive graphics + Step-by-step |

Home Medical Devices: Zero Cognitive Load Design
Safety warnings for sleep apnea machines: Elderly users are prone to overlooking risks such as overfilling the water tank or mask leakage.
Culturally adaptive design: The instruction guide uses wordless illustrations with a "prohibition symbol + water droplet" to convey the message.

· Brand Differentiation Expression
Achieving brand differentiation in the design of medical device warning labels is not merely about adding a logo. Instead, it involves systematically integrating brand DNA into safety functions, creating a unique user experience within the compliance framework.
Core Dimensions of Brand Differentiation:
| Dimension | Traditional Design | Brand Differentiation Strategy | User Value |
|---|---|---|---|
| Visual Identity | Standard red/yellow warning colors | Extended brand color palette (e.g., gradients, soft tones) | Reduces anxiety, enhances trust |
| Interactive Feedback | Single-tone beep alarms | Brand-specific sound effects/tactile encoding | Improves warning recall and association |
| Emotional Connection | Functional warning text | Contextual language + brand IP integration | Strengthens perception of brand empathy |
| Technical Approach | Static labels | Proprietary interactive technology (e.g., AR projection) | Establishes perception of technical leadership |

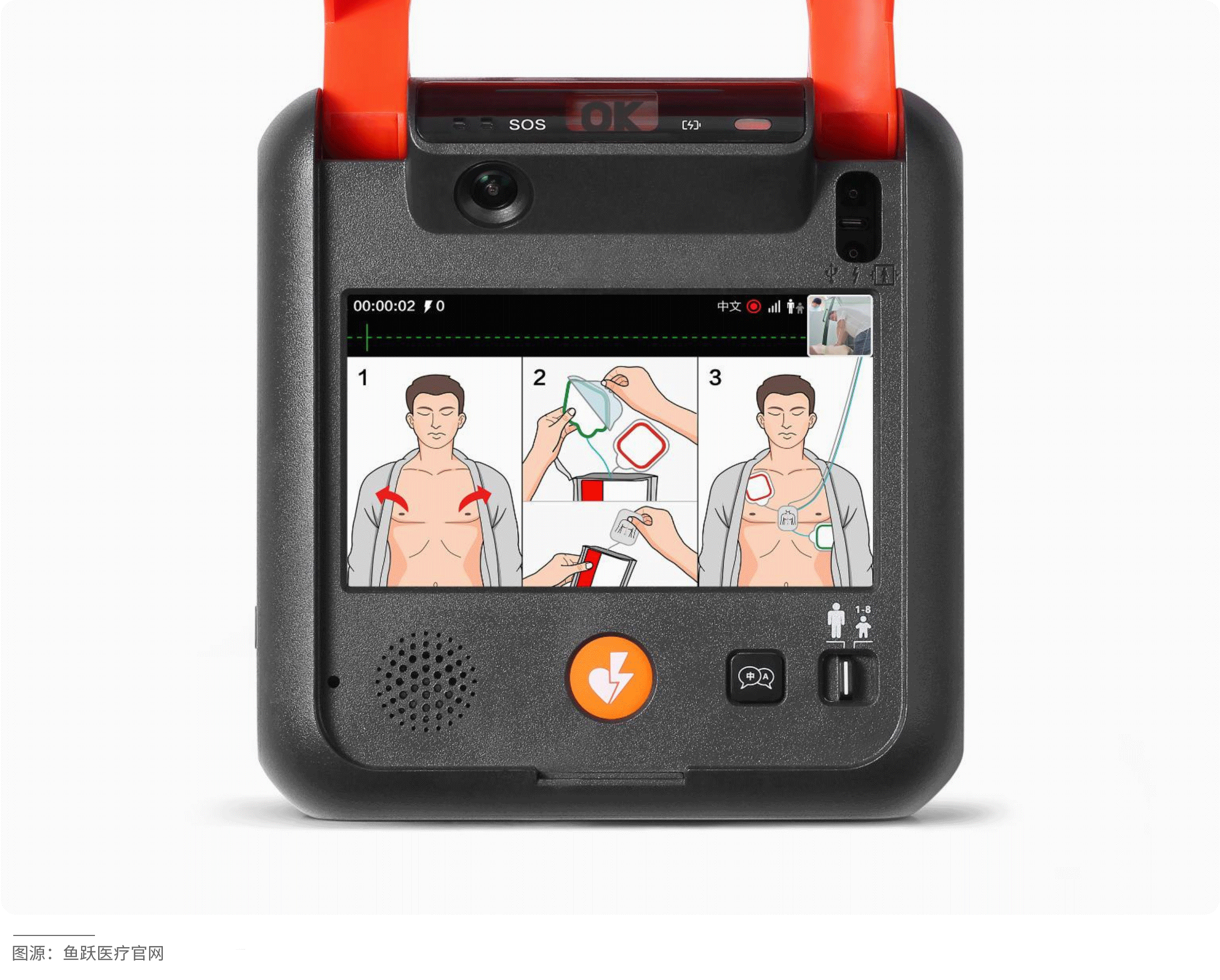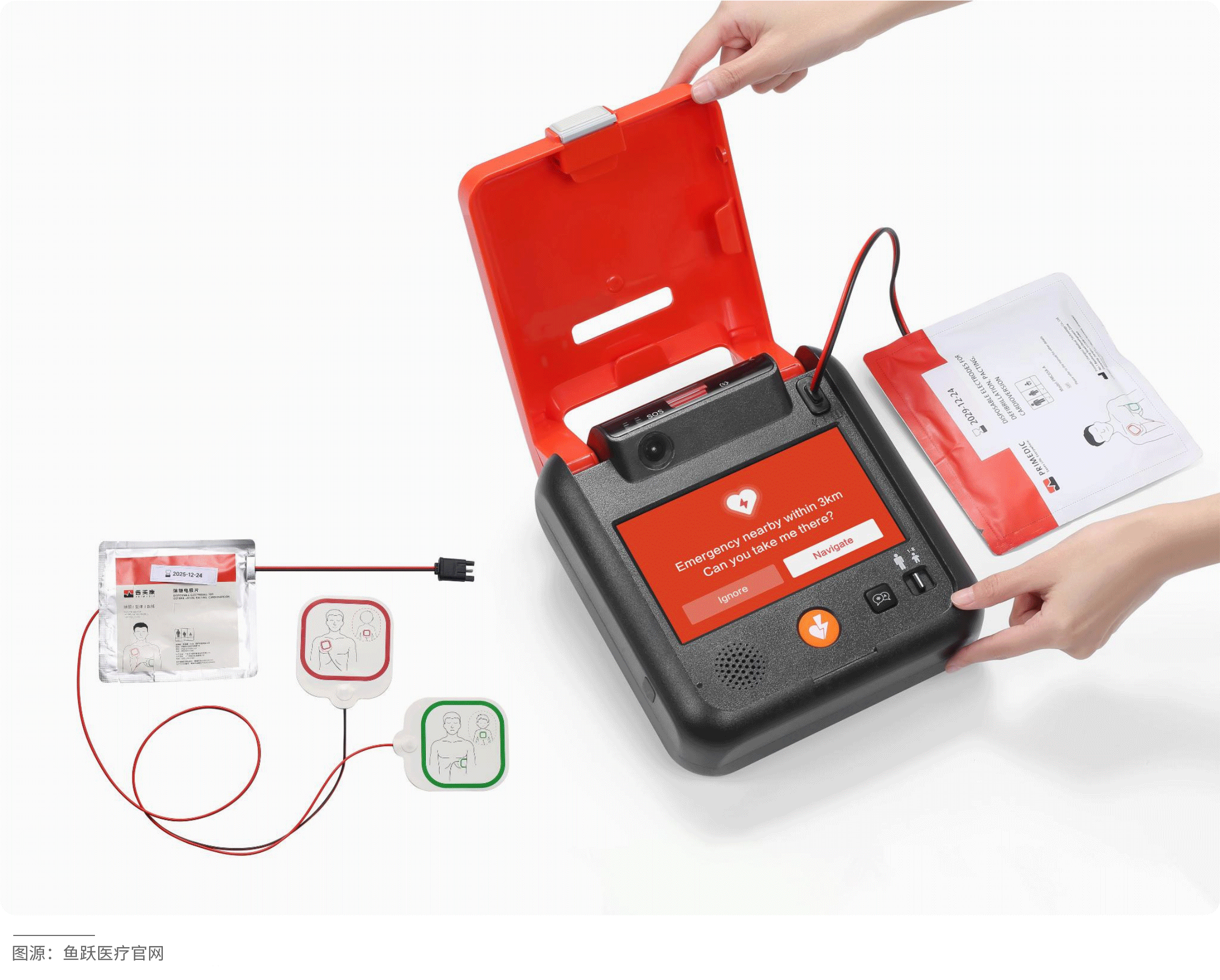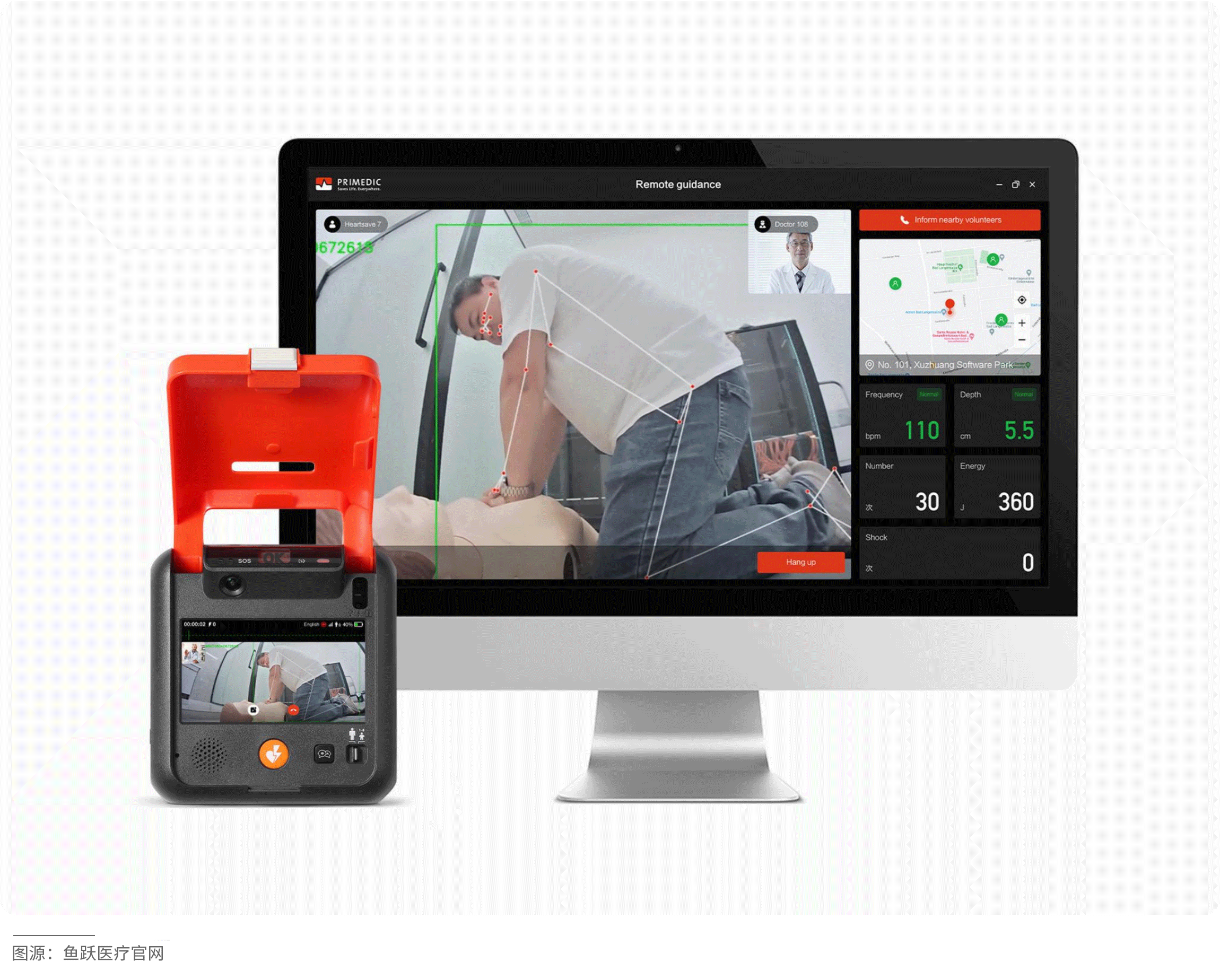
The HeartSave 7 is an automated external defibrillator (AED) that enables non-professionals to quickly and accurately assist patients experiencing sudden cardiac arrest. Equipped with a camera, it can clearly capture rescue footage and upload it in real-time to an emergency management platform, allowing doctors to instantly access first aid data and provide targeted remote guidance to improve rescue success rates. Leveraging artificial intelligence technology, the device intelligently analyzes the rescuer's actions and provides voice prompts to facilitate effective rescue operations. It also supports SOS calling to shorten response times and optimize resource allocation.
(Industrial Design Principles for Medical Product Warning Labels)
The industrial design of medical device warning labels must ensure safety and compliance while also achieving clear information communication, adaptation to complex usage scenarios, and meeting human-machine interaction needs. The following are the core design principles for medical devices:
· Compliance and Standardization
Core regulatory documents:
"Regulations for the Supervision and Administration of Medical Devices" (2021 Revised Edition)
"Provisions for Medical Device Instructions and Labeling" (Order No. 6 of the NMPA)
GB/T 191-2008 "Pictorial Marks for Packaging-Storage and Transportation"
YY/T 0466.1-2016 "Symbols to Be Used With Medical Device Labels, Labeling, and Information to Be Supplied"
Requirements from competent authorities:
All labels for medical devices sold/used in China must be filed or registered with the National Medical Products Administration (NMPA).
Imported medical devices must simultaneously display labels in Chinese, and the content must not conflict with the labels of the country of origin.
Guidelines for the use of warning symbols:
Prioritize the use of the YY/T 0466.1 symbol library (a total of 58 standard symbols), such as:
⚠ indicates a warning (must be accompanied by text, e.g., ⚠ + "Caution: High Temperature")
Thermometer + number indicates storage temperature
Umbrella symbol indicates moisture protection
Prohibited ambiguous symbols: Avoid using symbols not adopted by Chinese standards (e.g., the EU CE mark must not be used alone).
· Principle of Cognitive Efficiency: The 0.5-Second Recognition Rule
Visual Hierarchy System:
| Level | Response Time Requirement | Design Requirements |
|---|---|---|
| Emergency | <0.3 seconds | Red flashing (3Hz) + 95dB pulsed tone (2000Hz frequency) + Haptic vibration (200Hz) |
| Warning | <0.8 seconds | Yellow static symbols (contrast ratio ≥7:1) + Text pop-up |
| Caution | <1.5 seconds | Blue background + Icons (compliant with ISO 7000 database) |
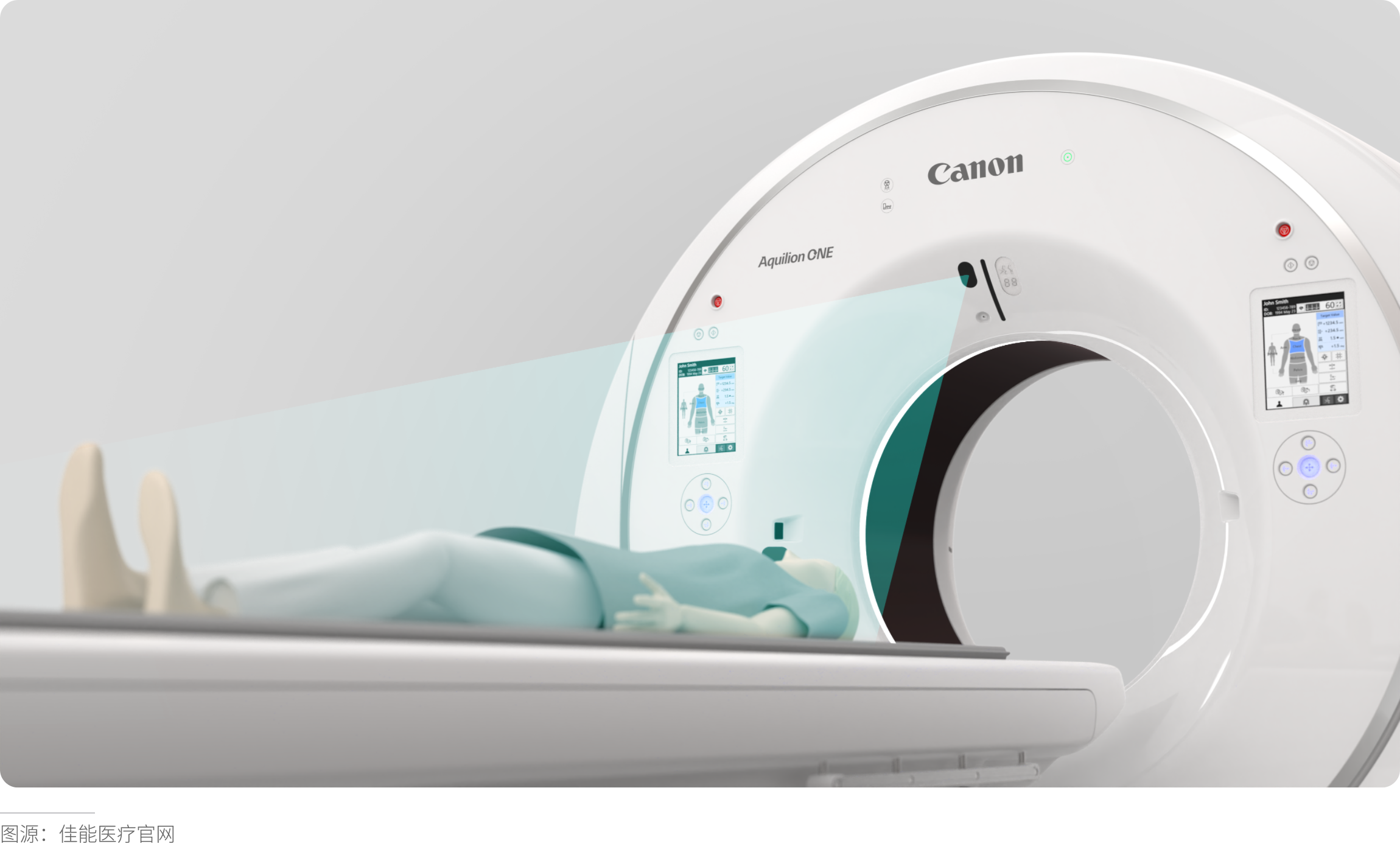
Eye-Tracking Optimization: CT Console Warning Layout
Original Design: Warning information was dispersed across 5 areas, with an average recognition time of 2.4 seconds.
Optimized Solution: Implemented an F-shaped visual heat zone layout, concentrating critical warnings in the top-left 1/3 of the screen.
Result: Recognition time reduced to 0.7 seconds, and correct emergency operation rates increased by 89%.
· Environmental Adaptation Principle: Full-Scenario Reliability Design
Comparison of Mainstream Processes:
| Process Type | Representative Materials | Advantages | Limitations |
|---|---|---|---|
| Laser Etching | Metal (Stainless Steel/Titanium), Ceramics | Permanent marking (lifespan > device cycle), High precision | High cost (+ RMB 5-15 per device) |
| Screen Printing/Pad Printing | Epoxy Resin Ink | Low cost, Multi-color options | Wear resistance < 5000 cycles (ISO 18115) |
| In-Mold Decoration (IMD) | Medical-grade ABS/PC | Integrated molding, Prevents detachment | Limited color options (requires pre-made color masterbatch) |
| Label (Adhesive Sticker) | PET Film + Acrylic Adhesive | Easy replacement, Multi-language adaptation | Temperature resistance < 80°C (non-sterilizable) |
| PVD Coating | Titanium / Chromium Alloy | High hardness (HV≥2000), Corrosion resistant | Requires vacuum equipment, suitable for mass production |

· Accessibility and Inclusive Design

(Epilogue)
From the perspective of industrial design, medical warning labels have transcended the traditional concept of "warning tags" and evolved into an intelligent safety network for human-machine symbiosis. Through the deep integration of materials science, interaction design, and artificial intelligence, WYYL Industrial Design is building a more proactive and inclusive medical safety ecosystem.
Frequently Asked Questions
1. Medical Device Product Classification
Medical devices are classified into the following categories based on their risk level:
Class I Medical Devices: Medical devices for which routine management is sufficient to ensure their safety and effectiveness.
Class II Medical Devices: Medical devices for which safety and effectiveness need to be controlled.
Class III Medical Devices: Medical devices that are implanted in the human body; used to support or sustain life; or pose a potential danger to the human body, and for which safety and effectiveness must be strictly controlled.
2. Classification of Medical Device Product Standards
International Standards: Technical specifications developed by international organizations such as the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC), applicable to the research, development, production, and circulation of multinational medical devices.
National Standards: Standards that require unified technical requirements nationwide. For technical requirements that need to be unified across the country, national standards shall be formulated.
Industry Standards: Standards that specify unified technical requirements within a specific industry. These standards apply to medical device products within that specific industry.
Registered Product Standards: Standards formulated according to the technical requirements during the medical device registration process. These standards are typically used to ensure the safety and effectiveness of the medical device.
3. Required Labeling for Medical Device Products
Registration Certificate Markings: After a medical device is registered with the national medical products authority, a registration certificate is issued. The registration certificate markings include the registration certificate number, validity period, and other information.
Product Identification: Used to identify the name, model, manufacturer, production date, and other information of the medical device.
Packaging Labeling: Relevant information on the medical device's packaging box, bag, or container, such as product name, specification model, batch number, production date, etc.
Instruction for Use Labeling: Content that should be included in the instructions for use of the medical device, such as usage method, precautions, maintenance, etc.
Warning Labels: Warning information indicating the hazards and prohibitions associated with the medical device, to remind users of correct usage and avoid dangers.
Cleaning and Disinfection Labels: Labels indicating the cleaning and disinfection requirements of the medical device to ensure its safety and reliability.
Packaging Material Identification: Information identifying the material and quality standards of the medical device's packaging materials to ensure the safety and protective performance of the medical device.
To obtain the latest version of the China Medical Device Labeling Guide or the UDI implementation details, please visit the official website of the National Medical Products Administration (NMPA): NMPA Official Website.
















