What certifications are required for medical product housing?

In the design and manufacturing of medical products, the housing not only serves to protect internal precision components and enhance product aesthetics, but its safety and compliance are also directly tied to user experience and market access. As an industrial design company, WYYL Design deeply understands the certification framework required for medical product housing, which is crucial to ensuring compliance with regulatory requirements and enhancing market competitiveness. The following analysis expands on core certification dimensions, providing compliance guidance for the design and production of medical product housing.
Current Outline:
· Understanding Medical Product Housings
· Global Core Certification Systems
· Performance Certification and Design Implementation
(Understanding Medical Product Housings)
Medical product housing refers to the external structural components used to protect, encapsulate, or support medical devices or equipment. It typically serves as a physical barrier between the medical device and the external environment (such as users, patients, liquids, microorganisms, etc.). During the design process, Universal Gravity Design takes into account the unique requirements of the medical industry, user needs, functional implementation, and regulatory standards to ensure safety, reliability, and compliance, meeting the standards for shipment.
· Necessity of Medical Product Housings
Medical environments can be unpredictable. Temperature fluctuations, exposure to liquids, and the presence of contaminants such as dust and bacteria can significantly impact the functionality of medical devices. Additionally, factors such as the actual handling of devices by multiple users, the risk of impact or dropping during transportation, and stringent hygiene requirements make robust medical device housings essential.

(Global Core Certification Systems)
The regulatory requirements for medical device registration vary across countries, exhibiting both differences and similarities. The United States and the European Union, as major regulatory forces in the medical device market, have particularly stringent registration and certification requirements. The US FDA conducts meticulous reviews for the market registration and certification of medical devices, especially for the PMA certification of Class III devices, which requires companies to provide comprehensive clinical trial data and production standards. In the EU, following the implementation of the MDR, higher demands have been placed on pre-market approval and clinical evidence for high-risk and innovative devices, leading many previously approved products to require re-certification. Furthermore, Japan's PMDA regulates based on risk classification, with clear regulatory requirements ranging from product notification to official approval. In developing countries overseas, the overall rules are relatively similar; however, most countries simplify the process for products that have already obtained FDA or CE certification, though some nations still set their own entry barriers.
· Medical Device Certification Systems by Country
| Country/Region | Regulatory Authority | Classification and Registration Requirements |
|---|---|---|
| China | NMPA | The NMPA is China's regulatory agency for drugs and medical devices, responsible for the registration, supervision, and management of medical devices. Medical devices sold in China must obtain NMPA registration certification to ensure compliance with national regulations and technical requirements. The NMPA registration process includes technical evaluation, clinical trials, and quality management system audits to ensure the safety and effectiveness of medical devices. Devices are classified into Class I, II, and III based on risk level, overseen by municipal, provincial, and national-level medical products administrations respectively. |
| United States | FDA | The FDA is the U.S. food and drug regulatory agency responsible for the supervision and approval of medical devices in the U.S. market. Medical devices are classified into Class I, II, and III based on risk. Most Class II devices require a pre-market notification (510(k)), while Class III devices must submit a Premarket Approval (PMA) application and supporting documentation to the FDA before marketing. |
| European Union | CE Mark & National Competent Authorities | The CE mark is the unified certification mark for medical devices in the European Economic Area. Previously, certification under the MDD was less difficult, but regulation has tightened. The Medical Device Regulation (MDR) came into force in May 2021, classifying invasive medical devices into Class I, IIa, IIb, and III based on risk. The In Vitro Diagnostic Regulation (IVDR) came into force in May 2022, classifying non-invasive in vitro diagnostic (IVD) devices into classes A, B, C, and D from low to high risk. |
| Japan | PMDA | Medical devices are classified into Class I, II, III, and IV based on risk, regulated respectively through product notification (Class I), third-party certification (Class II), and approval by the Ministry of Health, Labour and Welfare (MHLW) (Class III, IV). |
| Other Developing Nations | Local Medical Products Authorities | Medical devices are generally classified into Class I, II, III (and sometimes IV), with overall relatively similar rules. Some countries in Latin America, Africa, and Southeast Asia simplify procedures as much as possible for products with existing FDA or CE certification, while some also recognize NMPA certification. |

· Cornerstone of Quality Management Systems: ISO 13485
ISO 13485 is a quality management system standard developed by the International Organization for Standardization (ISO) specifically for the medical device industry. It encompasses all aspects of medical device production and related services, including design and development, manufacturing, installation, after-sales service, and technical support.
As a quality management system standard for the medical device industry, ISO 13485 addresses the unique nature of medical devices as products crucial for saving lives, preventing, and treating diseases. Relying solely on the general requirements of ISO 9000 is insufficient for this sector. Therefore, ISO 13485 incorporates specific requirements for the medical device industry, imposing stricter controls on aspects such as product identification and process control. It sets dedicated requirements for the quality management systems of medical device manufacturers, significantly contributing to ensuring that medical devices are safe and effective. ISO 13485 certification is the most widely accepted standard for medical device manufacturers worldwide (e.g., in the United States, Japan, Canada, and the European Union).

· Global Compliance Integration: MDSAP
MDSAP certification is an acronym for the Medical Device Single Audit Program. This program is recognized and participated in by regulatory authorities from five countries: the U.S. FDA, the Australian TGA, Brazil's ANVISA, Canada's HC, and Japan's MHLW. By obtaining MDSAP certification, manufacturers can substitute certain audits and routine inspections in these countries, thereby facilitating easier market access. Due to the high requirements of MDSAP certification, companies that achieve it can typically demonstrate the high quality and safety of their medical device products.
Significance of MDSAP Certification:
Medical device manufacturers engage a third-party Notified Body (e.g., SGS, BSI, TÜV) to conduct the MDSAP audit. The medical device regulatory authorities of the participating countries (the five nations: United States, Australia, Brazil, Canada, Japan) can use this audit report as a basis for their regulatory decisions.
The level of recognition in relevant countries is as follows:
United States: Can replace routine FDA inspections (excluding FDA-for-cause inspections and PMA products).
Brazil: For Class III and IV medical devices, can replace ANVISA's pre-market GMP inspection and post-market routine inspections (excluding targeted inspections).
Japan: For Class II, III, and IV medical devices, can exempt on-site factory audits.
Canada: Since 2019, MDSAP has mandatorily replaced CMDCAS as the only pathway for Class II and higher devices to enter the Canadian market.
Australia: Can exempt TGA audits and supports the issuance and maintenance of TGA conformity audit certificates.
(Performance Certification and Design Implementation)
Performance certification for medical product housings aims to ensure they meet the functional, durability, environmental adaptability, and mechanical strength requirements for medical device use. Targeted testing must be conducted based on the product's intended use, usage environment, and the regulations of the target market.
· Biocompatibility Testing
Applicable Scenarios: Direct or indirect contact with the human body (e.g., surgical instrument housings, medical catheter housings, implantable device housings).
Test Items:
Cytotoxicity Testing: Medical device materials are contacted with cells to observe cell morphology, proliferation, etc., assessing the material's toxicity to cells.
Immunogenicity Testing: Evaluates the immune response of laboratory animals to the medical device, checking for potential allergenicity.
Skin Irritation Testing: The medical device is contacted with animal or human skin to observe whether it causes skin irritation.
Sensitization Testing: Assesses whether the medical device has sensitization potential by simulating contact in the use environment.
Design Implementation:
Material Selection: Prioritize the use of medical-grade materials (e.g., medical-grade ABS, PC, PEEK) and avoid additives that may leach harmful substances.
Surface Treatment: If coatings or printing are required, ensure they comply with biocompatibility requirements (e.g., heavy-metal-free inks, color masterbatches conforming to ISO 10993-5).
Risk Control: Conduct focused verification on high-contact-risk areas (e.g., interfaces in prolonged contact with the skin).

· Chemical Compatibility Verification
The housing must resist corrosion from disinfectants (such as alcohol, hydrogen peroxide) and drug residues. Some devices need to withstand gas residue penetration testing after ethylene oxide (EO) sterilization.
Test Items:
Composition Analysis: Content detection of heavy metals (e.g., lead, cadmium), residual monomers (e.g., bisphenol A, vinyl chloride), and additives (plasticizers, antioxidants) using methods like ICP-MS and GC-MS.
Leachables Testing: Analysis of leachables under simulated use conditions (e.g., different solvents, temperatures, durations), such as immersion in saline or alcohol, detected by HPLC.
Chemical Resistance: Evaluation of changes in appearance and mechanical properties after immersion in acids/bases or disinfectants (e.g., iodophor, alcohol) per standards like ASTM D543.
Design Implementation:
Material Verification: Conduct immersion tests with common disinfectants (e.g., isopropyl alcohol, hydrogen peroxide) to screen suitable materials (e.g., PPSU offers excellent chemical resistance).
Protection Design: For highly corrosive scenarios, a dual-layer structure can be adopted (inner layer for corrosion resistance, outer layer for protection).

· Mechanical Performance and Environmental Stability
Testing the structural strength and stability of the device to identify potential risks of mechanical damage; evaluating the performance and reliability of the device under various usage environments, including temperature, humidity, air pressure, and other conditions.
Test Items:
Mechanical Properties: Tensile strength, yield strength, elongation at break (assessing material toughness, ASTM D638); Impact strength (e.g., Izod impact test, ASTM D256); Compression/flexural strength (assessing deformation resistance).
Dimensional Accuracy: Tolerance ranges, fit precision (e.g., interface dimensions, ISO 8871).
Drop Resistance: Drop tests from different heights (simulating accidental drops during transportation or use, GB/T 14806).
Temperature/Humidity Cycling: Assessment of dimensional stability and changes in mechanical properties under high temperature (e.g., 50°C), low temperature (e.g., -20°C), and damp heat (e.g., 85% RH) conditions.
Aging Tests: UV aging, ozone aging (simulating environmental degradation during long-term use, ASTM G154).
Ingress Protection (IP) Rating: IPXX protection level testing (e.g., IP67 rating: completely dust-tight and protected against immersion in water up to 1 meter for 30 minutes, IEC 60529).
Design Implementation:
Structural Strength: Reinforce stress-bearing areas (e.g., adding ribs to screw posts) to prevent cracking during transportation.
Sealing Design: For devices requiring water resistance (e.g., those used outside operating rooms), implement IP67/IP68 solutions (e.g., two-shot molded seals).
Material Weather Resistance: Select high-temperature resistant materials (e.g., PEEK, which can withstand 250°C steam sterilization) for housings of devices requiring high-temperature sterilization.

· Flame Retardancy Certification
Applicable Scenarios: Medical electrical equipment housings (e.g., for patient monitors, surgical light housings), medical equipment used in public areas.
Test Items:
Horizontal/Vertical Burning Test: Assesses flame retardant rating (e.g., V-0 grade: extinguishes within 10 seconds after flame removal, with no dripping of flaming particles; V-2 grade: allows dripping particles that ignite cotton).
Glow-Wire Test: Simulates resistance to ignition when exposed to a high-temperature heat source (IEC 60695-2-10).
Design Implementation:
Prioritize Pre-certified Materials: Using UL Yellow Card recognized materials (e.g., Sabic LEXAN™ 940A, rated UL 94 V-0) can shorten testing cycles. Medical-grade flame-retardant PC/ABS (offering both chemical resistance and mechanical strength) is also suitable.
Reduce Heat Accumulation: Incorporate heat dissipation vents (aperture size must comply with IEC 60601-1 electrical safety requirements, e.g., <12mm).
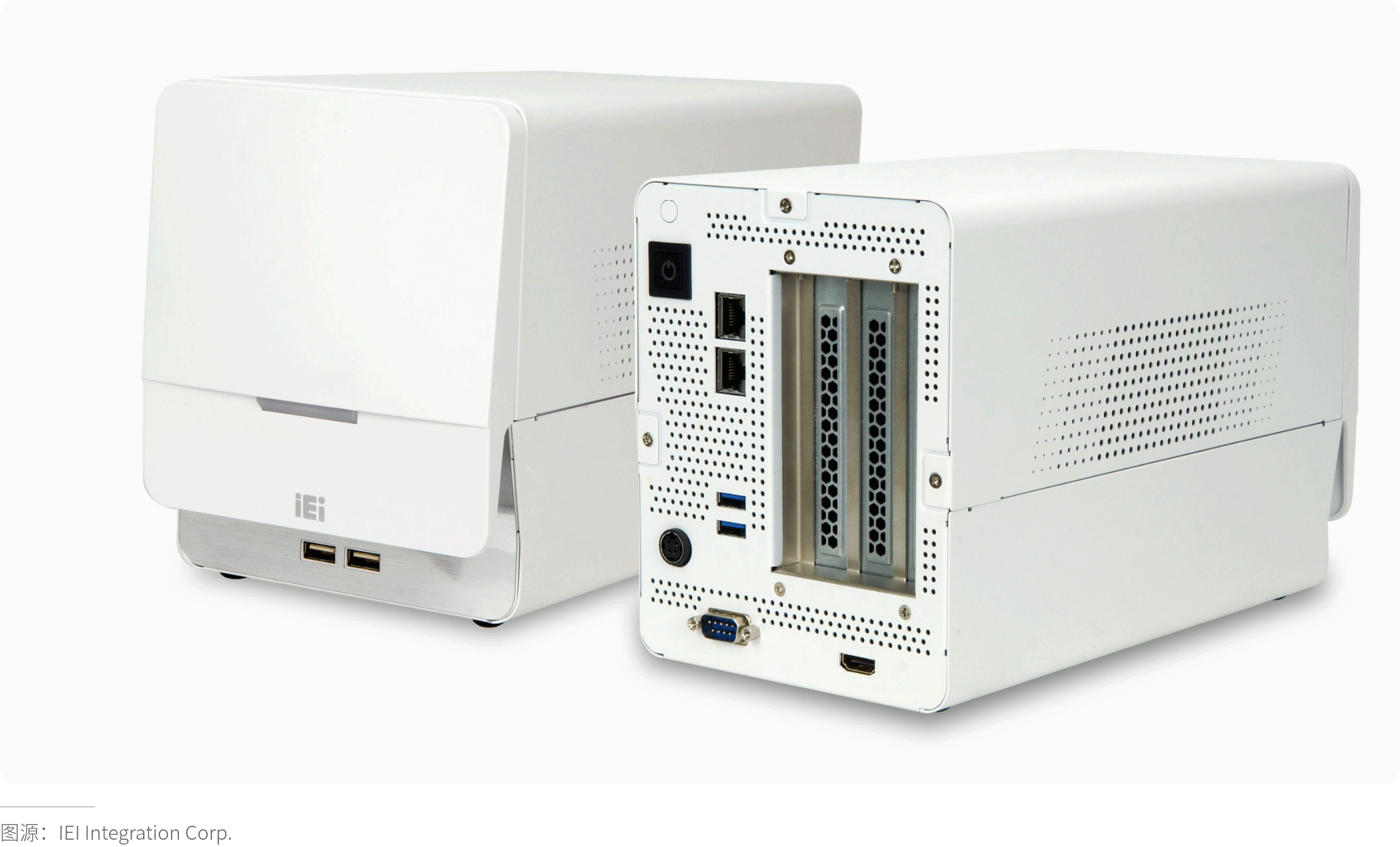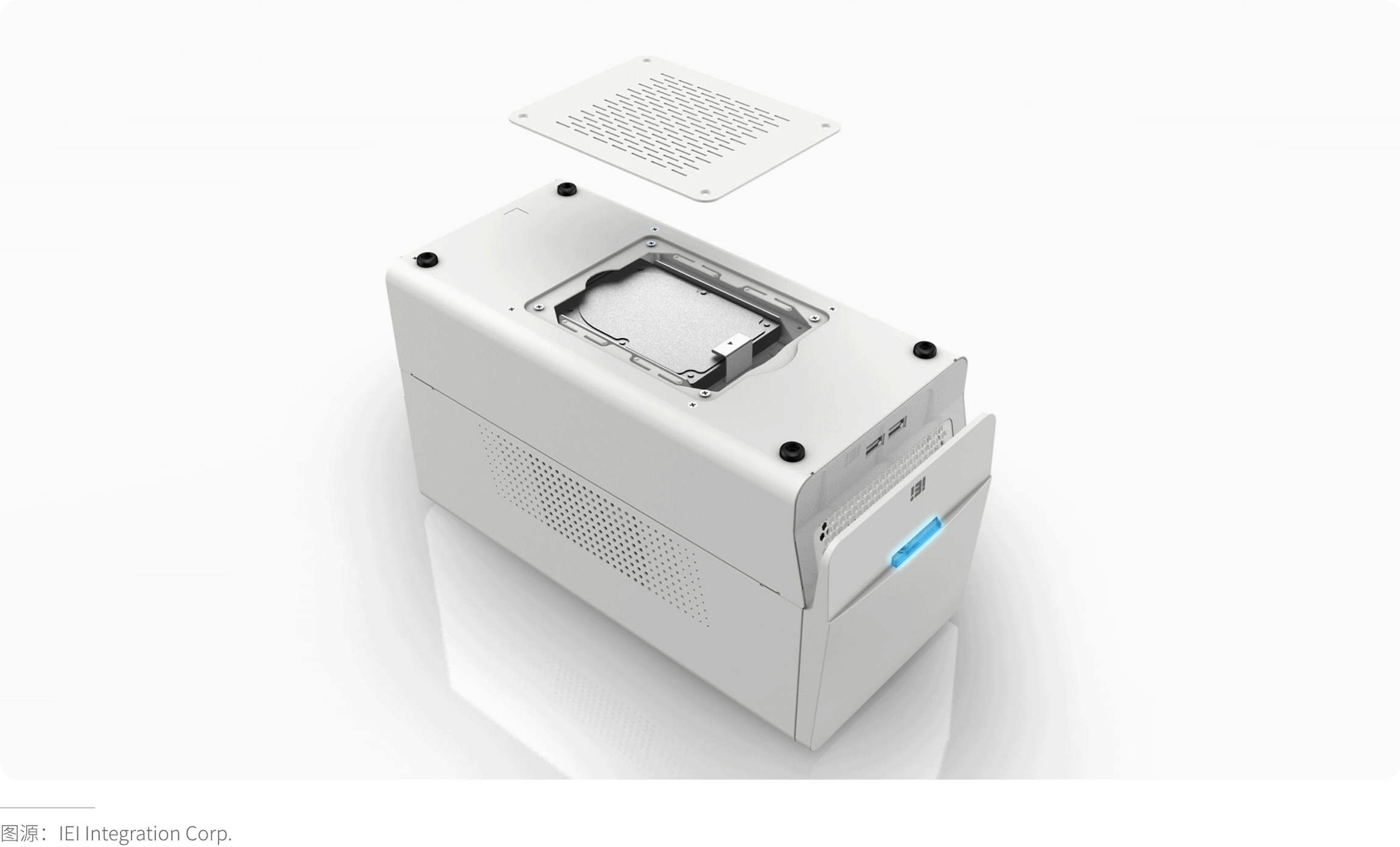
· Sterilization Tolerance Verification
Applicable Scenarios: Disposable/reusable housings that require sterilization before use (e.g., surgical instrument trays, medical packaging housings).
Test Items:
Sterilization Tolerance: Evaluating the effects of sterilization processes (e.g., EO gas, gamma radiation, high temperature and pressure) on the housing material (such as discoloration, embrittlement, dimensional changes).
Sterilization Residues: Testing for EO residue levels (ISO 10993-7); verifying radiation dosage (ensuring sterilization efficacy without excessive exposure).
Design Implementation:
Tolerant Structures: For housings requiring repeated sterilization (e.g., surgical instruments), prioritize high-temperature resistant materials (e.g., PEEK) and avoid snap-fit structures (which are prone to aging and cracking).
Sealing Design: Utilize two-shot molded gaskets or laser welding processes to ensure no leakage occurs after high-pressure steam sterilization (121°C/30min).

· Electromagnetic Compatibility (EMC) Design
Compliance with standards such as EN 61000-6-3 (Emissions), EN 61000-6-1 (Immunity), and YY 0505 requires the housing to possess electromagnetic shielding capability (e.g., metal coatings, conductive plastics) to prevent interference with internal circuits or external equipment.
Test Items:
Use electromagnetic compatibility testing equipment, such as electromagnetic radiation testers and electromagnetic immunity testers, to evaluate the product in specific electromagnetic environments. By simulating different electromagnetic interference sources and intensities, the product's EMC performance is verified against the requirements of relevant standards.
Design Implementation:
Shielding Design: For equipment sensitive to high-frequency interference, employ metal housings or add conductive coatings (e.g., nanosilver coating).
Structural Optimization: Minimize gaps and apertures (e.g., using conductive foam for sealing) to avoid electromagnetic leakage.
Filter Integration: Reserve space for filter installation at housing interfaces.
(Conclusion)
For Universal Gravity Design, a profound understanding of the certification framework for medical product housings is crucial to successfully bringing clients' products to market. Compliance must be integrated into every stage, from material selection and structural design to production processes. By planning the certification pathway early in the design phase, it is possible not only to reduce the costs of subsequent modifications but also to enhance the product's market competitiveness, thereby creating greater value for clients.
Frequently Asked Questions
1. What are the six key certificates to verify when purchasing a medical device product?
(1) Medical Device Manufacturing Enterprise License (for Class II and III medical device manufacturers);
(2) Medical Device Product Registration Certificate;
(3) Medical Device Distribution Enterprise License of the distributor handling the product (refers to distributors of Class II and III products, excluding the 13 specific Class II products);
(4) Product Qualification Certificate;
(5) 3C Certification Certificate and Mark (for medical device equipment listed in the "Catalogue of Products Subject to Compulsory Certification");
(6) EMC Certificate (for medical electrical equipment).
2. What is ISO 13485?
ISO is the International Organization for Standardization. It is an independent, non-governmental international organization with a membership of 174 national standards bodies. These members work to develop official International Standards, which are: voluntary; consensus-based; and market-relevant.
ISO 13485 is an internationally recognized Quality Management System (QMS) standard specifically designed for the medical device industry. It outlines standardized requirements for all organizations involved in the design, production, installation, servicing, and/or manufacturing of medical devices. This system provides these organizations with a framework to ensure their products and services consistently meet customer and regulatory requirements. ISO 13485 was first published in 1996; the latest version, ISO 13485:2016, took effect in 2016.
3. Conditions for Applying for Quality Management System Certification Registration
(1) The applicant organization must hold a business license or documents proving its legal status.
(2) It must have obtained a production license or other required qualification permits (where mandated by national or departmental regulations);
(3) The products covered by the QMS applied for certification must conform to relevant national standards, industry standards, or registered product standards (enterprise standards). The products should be finalized and produced in batches.
(4) The applicant organization must have established a management system conforming to the standard it is applying for. Medical device manufacturing and distribution enterprises must also meet the requirements of the YY/T 0287 standard. For enterprises manufacturing Class III medical devices, the QMS must have been in operation for at least 6 months. For enterprises manufacturing and distributing other products, the QMS must have been in operation for at least 3 months. At least one full internal audit and one management review must have been conducted.
(5) Within one year prior to submitting the certification application, the applicant organization's products must not have had any major customer complaints or quality incidents.
















