CACLP 2025 Concludes Successfully | A Glimpse into the On-Site Spectacle!

From March 22 to 24, 2025, the 22nd China International Congress on Laboratory Medicine and Clinical Laboratory Exposition (CACLP) and the 5th China International IVD Upstream Raw Materials & Supply Chain Expo (CISCE) with the theme "Driving the Healthy Development of the IVD Industry with New Forces" were successfully held at the Hangzhou International Expo Center. As one of the most influential global events in the in vitro diagnostics (IVD) industry, it attracted over 1,000 exhibitors and more than 50,000 professional visitors from around the world to gather in Hangzhou, discussing cutting-edge technologies, sharing innovative achievements, and promoting the booming development of the IVD industry.
By harnessing innovative forces to drive the healthy developmwnt of the IVD industry.
With the rapid advancement of technologies like artificial intelligence, big data, and the Internet of Things, intelligent diagnostics emerged as the highlight of this exhibition. Many companies showcased AI-driven automated diagnostic equipment, smart laboratory management systems, and remote diagnostic solutions, drawing significant attention. Let’s explore the key products from leading companies.

Danaher is a global leader in life sciences and diagnostics innovation, holding industry-leading positions in multiple core business areas, particularly in life sciences, diagnostics, and environmental and applied solutions. It is renowned for its unique business model and management philosophy, employing the Danaher Business System (DBS), a management strategy that combines lean production and continuous improvement.
In addition to booth 2-E0601, its subsidiary Cytiva also had a booth in Hall 4. At booth 2-E0601, the Danaher Group and more than ten of its operating companies exhibited together, including Beckman Coulter Clinical Diagnostics, Leica Biosystems, Cepheid Molecular Diagnostics, Radiometer, Mammotome, SCIEX, Leica Microsystems, Beckman Coulter Life Sciences, Agela & Phenomenex, IDT, Molecular Devices, and Abcam. Most of its medical devices feature modular designs to reduce laboratory space usage, with a focus on human-machine interaction to minimize manual steps.





Mindray is a leading Chinese high-tech medical device manufacturer and a global innovator in medical equipment. Since its establishment in 1991, Mindray has been dedicated to the R&D and manufacturing of clinical medical devices, with products spanning four major fields: patient monitoring and life support, clinical laboratory testing and reagents, digital ultrasound, and radiological imaging. It aims to bring medical electronics that perfectly balance performance and price to every corner of the world.
The launch of the MT 8000S Standalone Intelligent Sample Processing System signifies Mindray’s extension of digital intelligence capabilities to the front-end of sample processing. It also reflects Mindray’s commitment to an open, patient-centric "equipment + IT + AI" ecosystem in IVD. Additionally, the newly released H-120 Hemoglobin A1c Analyzer further demonstrates Mindray’s focus on clinical needs and its vision to build a "whole blood ecosystem." With a User-Centered Design philosophy at its core, Mindray designs intelligent, lightweight, and adaptable devices tailored to diverse clinical scenarios.






Maccura Biotechnology was founded in 1994 and specializes in the R&D, production, and related services of in vitro diagnostic products. Maccura has established a full industry chain layout, from biological raw materials to medical laboratory products and professional services. It possesses the systematic expertise to develop and manufacture IVD equipment, reagents, calibrators, and quality controls. Its products cover various technological platforms, including biochemistry, immunology, rapid testing, hematology, molecular diagnostics, and pathology.
Centered on the concept of the "Intelligent Laboratory," Maccura showcased a range of new products, including a fully automated urine workstation and a new slide reader, offering comprehensive, efficient, and intelligent total solutions. Serialization, systematization, automation, and autonomy—Maccura's product design revolves around these four dimensions, emphasizing comprehensiveness and intelligence. While advancing systematization, serialization, automation, and autonomy, the company has also developed a family-style design language across its products, all aimed at enhancing the user experience and making diagnostics simpler.





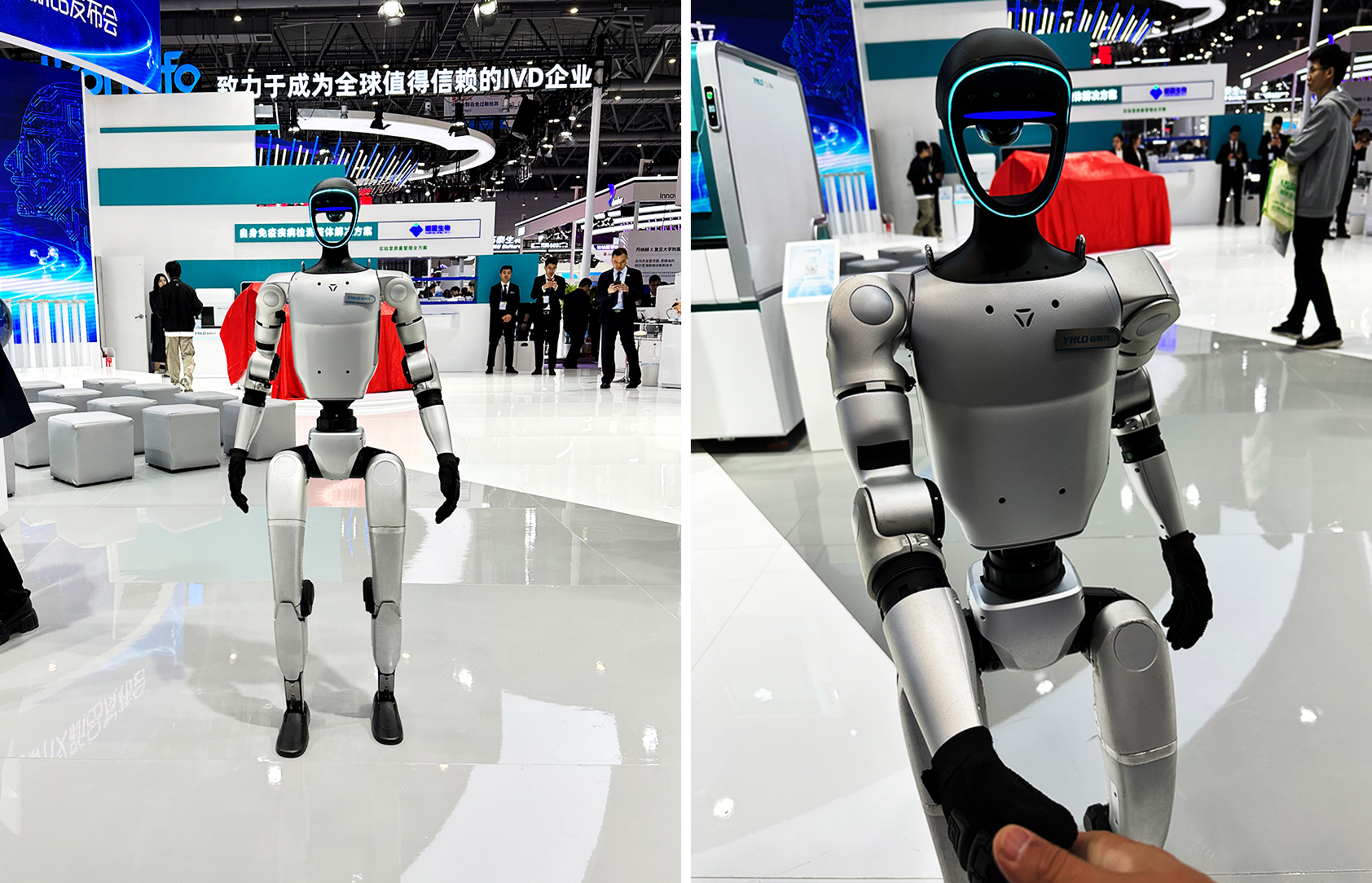
YHLO was established in 2008 and focuses on R&D and innovation in the IVD field. It currently has five major IVD technology platforms: chemiluminescence, biochemical testing, indirect immunofluorescence, immunoblotting, and colloidal gold immunochromatography. Its products cover business areas such as autoimmunity, infectious immunity, reproductive health, liver disease testing, respiratory testing, and cardiovascular disease testing.
The company held a new product launch for its fully automated biochemical analyzer, featuring multiple immunoanalyzers, biochemical-immuno integrated automation lines, and all-scenario smart ecosystem solutions. In terms of design philosophy and appearance, YHLO pursues "simplicity, sustainability, and innovation." Its medical product designs are clean and straightforward, allowing healthcare professionals to quickly adapt and focus on core tasks. The green color scheme aligns with the company's brand image while conveying an eco-friendly and harmless message. Innovation permeates technology, appearance, and functional details, giving the products a unique soul.




URIT Medical is a first-tier Chinese manufacturer, supplier, and service provider of medical diagnostic products, with a "National and Local Joint Engineering Laboratory for Immunoassay Reagents" accredited by the National Development and Reform Commission. For forty years, URIT has forged ahead, dedicated to health through laboratory testing. The company keenly follows trends in medical technology innovation and addresses clinical diagnostic needs, developing eight major product series, including hematology analyzers, urine analyzers, and biochemical analyzers.
URIT's booth, themed "Starlight Shines, Intelligent Testing Knows No Bounds," became the focal point of Hall 2. A dome of stars, created with dynamic holographic particles interweaving into a flowing galaxy, symbolized URIT's endless exploration in the field of precision medicine. This design not only highlighted the brand's technological DNA but also conveyed an attitude of "guarding life with reverence." URIT's industrial design for medical equipment distinctly combines innovation with simplicity, emphasizing ease of operation and user experience while paying attention to detail and ergonomics. Through these designs, URIT not only enhances product competitiveness but also provides efficient and reliable solutions for the healthcare industry.




Triplex International was founded in 2007. Centered on its self-innovated "Hard Substrate Protein Chip Detection Technology Platform," the company specializes in the R&D, production, sales, and service of high-throughput rapid testing products in the medical diagnostics industry. It holds a leading domestic position in the field of protein chips and detection instruments. Triplex possesses multiple technology platforms, including microarray protein chip technology, electrochemiluminescence technology, molecular diagnostics, biological raw materials technology, fully automated detection instrument technology, and integrated wearable biosensing technology. Its products are primarily used for the differential diagnosis and treatment monitoring of diseases such as tumors, metabolic diseases, cardiovascular diseases, infections, and autoimmune disorders.
During the exhibition, Triplex's booth design, featuring a dominant tech-blue color scheme and a clean, elegant layout, attracted numerous visitors. The fully automated electrochemiluminescence immunoassay platform and several innovative products were displayed. Staff conducted live demonstrations and interactive explanations, detailing the technical advantages and application scenarios of the products to clients. Triplex incorporates safety and reliability into its designs, utilizing high-quality materials and stringent production processes to ensure the safety and stability of the equipment under various usage conditions. The devices support multiple testing items, catering to diverse clinical needs.























